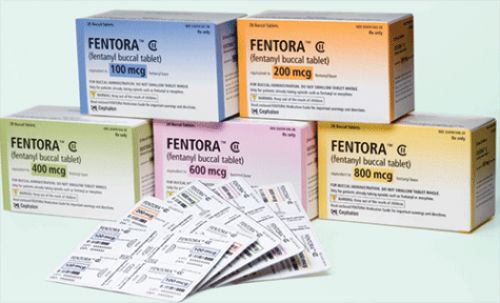
Provoking flashbacks to the controversy surrounding off-label OxyContin use, the FDA has issued an alert for health care professionals about pushing fentanyl buccal tablets (i.e., Fentora), since the high-powered opiate can be highly addictive and might actually
make you die if you take a little too much.
Fentora is a potent opioid pain medication that should be used only for the treatment of breakthrough pain in cancer patients who are receiving opioid treatment and who have become tolerant. Of particular interest to family physicians, the FDA warns against using Fentora for any short-term pain, such as headaches or migraines, sports injuries, or postoperative pain.
So here we have a warning about powerful opiates being prescribed by doctors to treat short-term aches and pains. I can hear lawyers scurrying to compile class-action lawsuit briefs as I type this.