Emapunil: possible new nonaddictive anti-anxiety drug
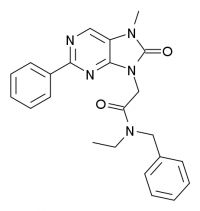 | | Emapunil, aka AC-5216 & XBD-173 |
From the Corpus Callosum blog: Those of us who watch the drug development pipeline have been pining for a nonaddictive anti-anxiety drug. Occasionally there are glimmers of hope. One candidate is emapunil, aka XBD-173 or AC-5216. In 2004, there was an article in the British Journal of Pharmacology about this. That article described promising findings, in rats and mice. Now, there is an article in Science that finally show some findings in humans. The abstract of the Science article follows...
Translocator Protein (18 kD) as Target for Anxiolytics Without Benzodiazepine-Like Side Effects: Science 24 July 2009: Vol. 325. no. 5939, pp. 490 - 493
Most antianxiety drugs (anxiolytics) work by modulating neurotransmitters in the brain. Benzodiazepines are fast and effective anxiolytic drugs; however, their long-term use is limited by the development of tolerance and withdrawal symptoms. Ligands of the translocator protein [18 kilodaltons (kD)] may promote the synthesis of endogenous neurosteroids, which also exert anxiolytic effects in animal models. Here, we found that the translocator protein (18 kD) ligand XBD173 enhanced {gamma}-aminobutyric acid-mediated neurotransmission and counteracted induced panic attacks in rodents in the absence of sedation and tolerance development. XBD173 also exerted antipanic activity in humans and, in contrast to benzodiazepines, did not cause sedation or withdrawal symptoms. Thus, translocator protein (18 kD) ligands are promising candidates for fast-acting anxiolytic drugs with less severe side effects than benzodiazepines.
Read the full post for more details from human trials and a history of research into emapunil.
Thanks TJ!
|

Recently @ DoseNation
|
|


















Further, in a trial on seventy healthy subjects for a week, the absence of signs of dependency does not indicate lack of potential for abuse of dependence longer term. A moot case is that of the opioid nasal spray butorphanol for migraine, initially unscheduled but reclassified at the maker’s request to US S4 - Australian S8, because of addiction incidence. It takes a while for the ADR reports to mount on experimental drugs.
The drug trial was sponsored by the manufacturer Novartis (Hirschler 2009), so a degree of cynicism about claims is merited. Independence of studies about experimental drugs is a moot point for all the experimental drugs.
Will the drug ever hit the shelf? The rumour on the science blogsites is that the patent has been bought by a Japanese pharmaceutical giant, with no intention to market.....
Source :findrxonline
The comments posted here do not reflect the views of the owners of this site.